Lawyer Fights With FDA on J&J Risperdal Court Papers
July 12th, 2013 // 1:50 pm @ jmpickett
 Latest FDA and cGMP Compliance News
Latest FDA and cGMP Compliance News
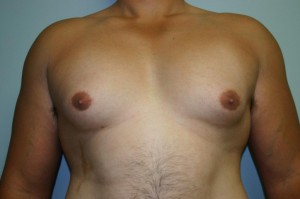
A lawyer is pushing FDA to try to obtain documents that supposedly show unpleasant side effects of the antipsychotic drug Risperdal. But the documents cannot be released to the public because of a court order. The papers were sealed by a judge in Pennsylvania in a case involving claims that Johnson & Johnson’s drug is responsible for causing gynecomastia, which is the development of breasts in men.
Since early in 2012, lawyer Stephen Sheller has tried to convince the agency that the documents need to be disclosed. He first filed a Citizen’s Petition that asked FDA to put a black box warning on the drug. He says that this was needed because there was not enough safety data for Risperdal. FDA is reviewing that petition but there is no other information on that at this time.
The heart of the case is a deposition by David Kessler, the ex-FDA commissioner. He submitted last fall a 100 page report as part of the lawsuit. He criticized J&J for promoting Risperdal for uses that are not approved by FDA. His report refers to other documents that are under an order of protection, Sheller claims.
New Webinar – The Hitchhiker’s Guide to 483s and Warning Letters
Sheller refers in a letter to a 2003 study review by Kessler in the Journal of Clinical Psychiatry. The study was funded by J&J, and the study showed that Risperdal treatment for the long term in children did not show any correlation between high levels of prolactin and gynecomastia in men and boys. Several of the authors of the study work at J&J.
Kessler says in the report that J&J did not present data in an entirely objective way. He alleged that the firm changed the results by ignoring adverse events that were reported in boys that are older than 10 years. He also claims that the study misled doctors and the health community.
Sheller wrote to FDA last month that Kellser was deposed on the topics in his report, including the criticism of the analysis. FDA, he said, has nothing stopping it from asking J&J for a copy of the transcript for a review.
Risperdal is a controversial drug for Johnson & Johnson. It has been sued over how it marketed the drug, and it is working with DOJ on a potential $2 billion settlement that could include criminal charges.
J&J states that a lot of clinical data has been given to FDA about use in children, such as internal reports, retrospective studies, post marketing studies, and a summary of relevant literature, according to a J&J spokesperson.
The spokesperson stated that the information was given after a request from FDA after Sheller filed his Citizen’s Petition. J&J gave a response to the request and it confirmed that it had given all data that had been requested for Risperdal.
Sheller thinks that enough has not been done. He thinks FDA may not be familiar enough with the documents that were filed in the court, especially the ones that were filed under protective order, including the deposition by Kessler.


