FDA Set to Regulate Fecal Transplants
May 21st, 2013 // 2:23 pm @ jmpickett
Latest FDA and cGMP Compliance News
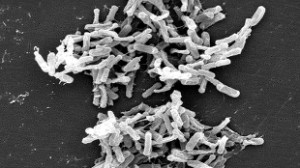
FDA has decided this week that fecal transplants are indeed biologic therapy, so this means that doctors and researchers will have to submit an IND application.
Researchers who want to perform fecal transplants, which is emerging as a good therapy to treat Clostridium difficile or C. diff infections, were told of this new requirement in February.
Is this a good move? Some researchers and doctors are worried that the new requirements will add a great deal of paperwork and a 30 day wait. This could restrict access to a very promising type of therapy. One researcher at the Virginia Commonwealth University told us that the ruling will impose a massive paperwork hurdle to getting needed therapies to patients with serious C. diff infections.
An infectious disease specialist named Judy Stone agrees. She has said this week that FDA should not include non-academic and non-research centers in the IND requirement, or organizations that only want to do a few procedures each month. If not, doctors are going to give more patients DIY, do it yourself treatments at home.
Other researchers think that while there will be added expense and paperwork, they understand the need for FDA to set new safety standards for the procedures.